REMIX study design
RHAPSIDO® (remibrutinib) was studied in the largest phase 3 clinical trial program for an oral CSU treatment1–3
REMIX-1 and REMIX-2 included over 900 adult patients with moderate to severe disease.1
925 adult patients with CSU were enrolled in 2 identical phase 3 randomized, multicenter, double-blind, placebo-controlled studies.1
Patients were randomized in a 2:1 ratio to receive either RHAPSIDO 25 mg or placebo, respectively, for 24 weeks during the double-blind treatment period and continued in a 28-week open-label treatment period, during which all patients received RHAPSIDO 25 mg BID.1
Second-generation H1 antihistamines started a minimum of 7 days before randomization through the end of the study. All patients were required to have a Weekly Urticaria Activity Score (UAS7) ≥16 (range 0–42), a Weekly Itch Severity Score (ISS7) ≥6 (range 0–21), and a Weekly Hives Severity Score (HSS7) ≥6 (range 0–21) for 7 days prior to randomization.1,4,5
Baseline characteristics
Baseline characteristics were well balanced between treatment arms.1,6
Similar baseline characteristics were seen in REMIX-1. Key subgroups in the clinical trials included CSU duration, angioedema history, CU-Index, prior exposure to anti-IgE biologics, and total IgE level.4-6
*Percentages may not total 100 because of rounding.6
aBMI is the weight in kilograms divided by the square of the height in meters.6
bHSS7 ranges from 0 to 21, with higher scores indicating greater severity.6
cISS7 ranges from 0 to 21, with higher scores indicating greater severity.6
dUAS7 ranges from 0 to 42, with higher scores indicating greater severity.6
eOf the 88 measurements that were missing in this trial, 65 were missing because the assessment was not performed in China.6
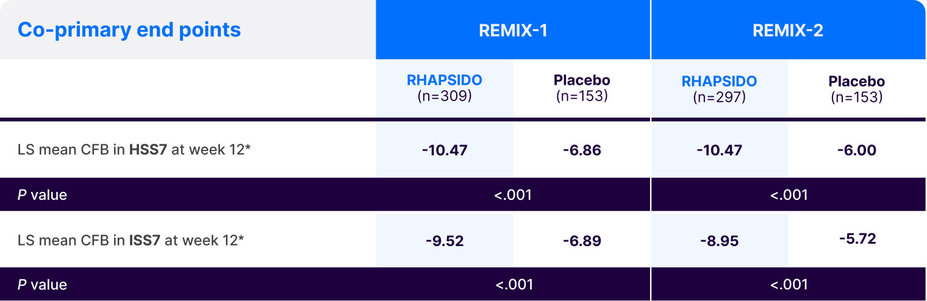
Study end points1,4,5
In the REMIX-1 and REMIX-2 trials, the co-primary end points were absolute change from baseline in HSS7 and ISS7 at week 12.1
*Multiple imputation techniques were implemented for missing data.1
Key secondary end points1,4,5
LS mean CFB in UAS7 at week 12
Proportion of patients with UAS7 ≤6 at week 2
Proportion of patients with UAS7 ≤6 at week 12
Proportion of patients with UAS7=0 at week 12
Cumulative number of angioedema-free weeks (AAS7=0) up to week 12