Demonstrated safety profile of RHAPSIDO® (remibrutinib)
Robust safety evaluation across 2 phase 3 trials up to 52 weeks1,2
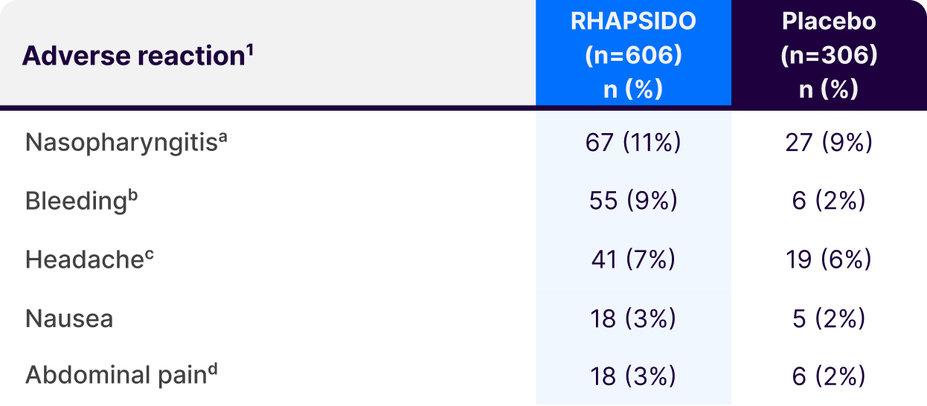
Adverse reactions with RHAPSIDO ≥3% and more common than placebo at week 241
aIncludes acute sinusitis, chronic sinusitis, nasopharyngitis, pharyngitis, pharyngitis streptococcal, rhinitis, rhinitis allergic, and upper respiratory tract infection.1
bAll bleeding events were mucocutaneous-related and included conjunctival bleeding, contusion, ecchymosis, epistaxis, gingival bleeding, hematoma, hematuria, hemorrhagic ovarian cyst, intermenstrual bleeding, petechiae, purpura, and urinary occult blood.1
cIncludes headache and migraine.1
dIncludes abdominal discomfort, abdominal distention, abdominal pain, and abdominal pain upper.1
Robust safety evaluation
868 patients with CSU were treated with RHAPSIDO across 2 phase 3 trials for up to 52 weeks1,2
In the pooled phase 3 trials, the safety profile of RHAPSIDO at 52 weeks was consistent with the safety profile at 24 weeks2
No severe bleeding reactions occurred1
No association between bleeding reactions and low platelet counts was observed1
Bleeding reactions led to discontinuation in 0.5% of RHAPSIDO patients vs 0% in placebo patients1
Why RHAPSIDO
RHAPSIDO offers an oral escalation option after antihistamines, with a demonstrated safety profile.1
NO laboratory monitoring required
NO boxed warning
NO contraindications
NOT a steroid
NOT an injection